News
Technetium-99 (99Tc)is a β-emitting radionuclide with a long half-life of 2.13×105 years.It predominately exists in the nuclear fuel cycle and in the environment as thepertechnetate anion (TcO4-), which exhibits highsolubility and high environmental mobility with transportation velocity beingalmost identical as that of groundwater. Tc(VII) complexes are volatile duringnuclear waste vitrification process, producing problems for the off-gas systemdesign. Another serious issue associated with this radioisotope is itscapability to greatly interface with the solvent extraction/back extraction processof uranium, neptunium, and plutonium through catalytic redox reactions,generating a notable barrier on the valence state control of these keycomponents in the fuel cycle. However, this task represents a huge challengegiven the combined extreme conditions of super acidity, high ionic strength,and strong radiation field.
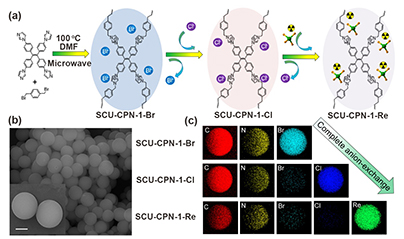
Figure 1. (a)Synthesis route of SCU-CPN-1 and its anion-exchange applications. (b) SEM image of SCU-CPN-1-Br, showingthe particle size is ca. 2 μm. (c) EDS mapping of SCU-CPN-1-Br,SCU-CPN-1-Cl, and SCU-CPN-1-Re, qualitatively indicative of the completeanion-exchange process.
Based on previous investigations(Journal of the American Chemical Society,2017, 139(42), 14873-14876;Environmental Science& Technology, 2017, 51,3471-3479; Environmental Science &Technology Letters, 2017,4(7), 316-322), Li et al. reports a facilely synthesized cationic polymericnetwork (CPN), SCU-CPN-1 (SCU = Soochow University) (Figure 1). This material has overcome the challenge with significantTcO4- uptake capabilities in five aspects (Figure 2): the fastest sorptionkinetics, the highest sorption capacity, the excellent selectivity, the mostpromising uptake performance from highly acidic solutions, and excellentradiation-resistance and hydrolytic stability among all anion sorbent materialsreported. In addition, this material is fully recyclable for multiplesorption/desorption trials, making it extremely attractive for wastepartitioning and emergency remediation.
The excellent TcO4-uptake capability is elucidated by X-ray absorption spectroscopy, solid-stateNMR measurement, and density functional theory analysis on anion coordinationand bonding. The DFT calculation illustrates that one side of the ReO4-(TcO4-) tetrahedron, consisting of three oxygenatoms, is almost parallel to the imidazole ring, forming relatively strong p-π interactions that leads to theface-to-face stacking structure, resulting in the binding energies of SCU-CPN-1with TcO4-/ReO4-(-8.91 kcal/mol /-8.63 kcal/mol) is significantly higher than that for SCU-CPN-1 withNO3- (-4.17 kcal mol-1).

Figure 2. (a)Sorption kineticsof SCU-CPN-1. (b)ReO4- sorption capacity of SCU-CPN-1 compared with other reported anion sorbents.(c) Radiation-resistance ofSCU-CPN-1, Purolite A530E, and Purolite A532E. (d) Reversibility of SCU-CPN-1 for removing ReO4- underthe condition of 3 M HNO3
SCU-CPN-1 not only exhibits excellent radiation-resistance and fastsorption kinetics that make it superior to traditional ion-exchange resins, butalso possesses excellent acidic and hydrolytic stability that MOFs don’t have.SCU-CPN-1 represents the best materials in Tc removal from spent fuel waste at present. This work is justpublished in Nature Communications (Nat.Commun., 2018, 9, 3007).
Prof. Shuao Wang andChengliang Xiao are the co-corresponding authors of the paper, and MissJie Li is the first author. The work was done at the State Key Laboratory ofRadiation Medicine and Protection, School for Radiological andInterdisciplinary Sciences (RAD-X) and Collaborative Innovation Center ofRadiation Medicine of Jiangsu Higher Education Institutions, Soochow University. Thanks for the help of Prof. Jing Chen and Chao Xu atTsinghua University in thedesign and operationof Tc sorption. Thanks for the help of Dr. Xing Dai atCenter of QuantitativeBiology and Medicine (Soochow University), Prof.Omar K. Farha and Dr. Peng Li atNorthwesternUniversity, and Prof. Thomas E. Albrecht-Schmitt atFlorida StateUniversity. Thanks for thefinancially support by the National Natural ScienceFoundation of China (21790370,21790374, U1532259, 11605118, U1732112), the Natural Science Foundation ofJiangsu Province (BK20150313), and the “Young Thousand Talented Program” inChina.
The original link:
Jie Li, Xing Dai, Lin Zhu, Chao Xu, DuoZhang, Mark Silver, Peng Li, Lanhua Chen, Yongzhong Li, Douwen Zuo, Hui Zhang,Chengliang Xiao*, Jing Chen, Juan Diwu, Omar K. Farha,Thomas E. Albrecht-Schmitt, Zhifang Chai, Shuao Wang*, 99TcO4- remediationby a cationic polymeric network, Nature Communications, 2018, 9, 3007.
https://www.nature.com/articles/s41467-018-05380-5